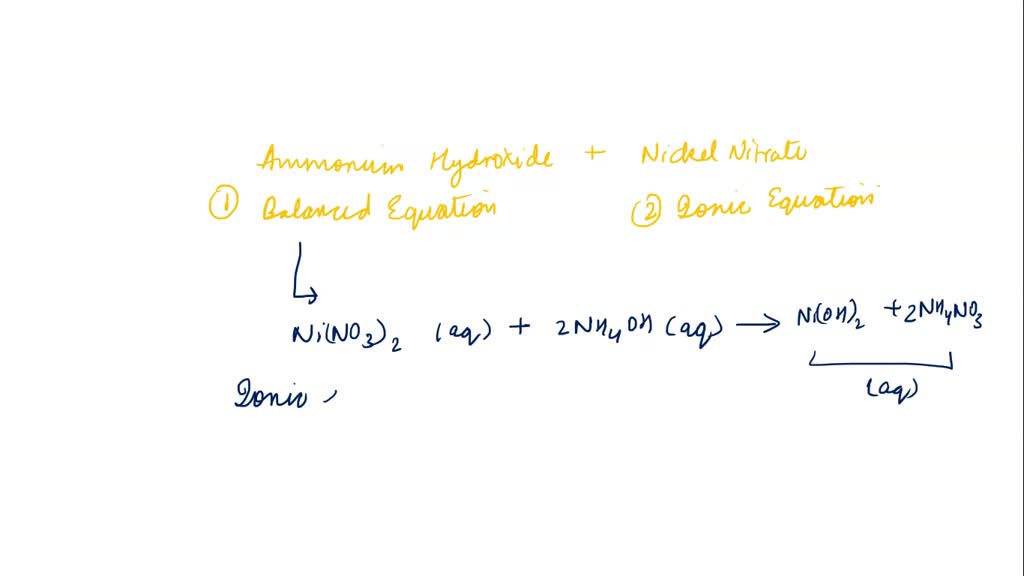
SOLVED: When an aqueous solution of NH4OH is mixed with an aqueous solution of Ni(NO3)2, a pale yellow precipitate forms. Write a balanced molecular equation for this reaction. Write the complete ionic

Scanning Electron Microscope image of Ni-BTC MOFs. (a) Ni-BTC Anl ; (b)... | Download Scientific Diagram
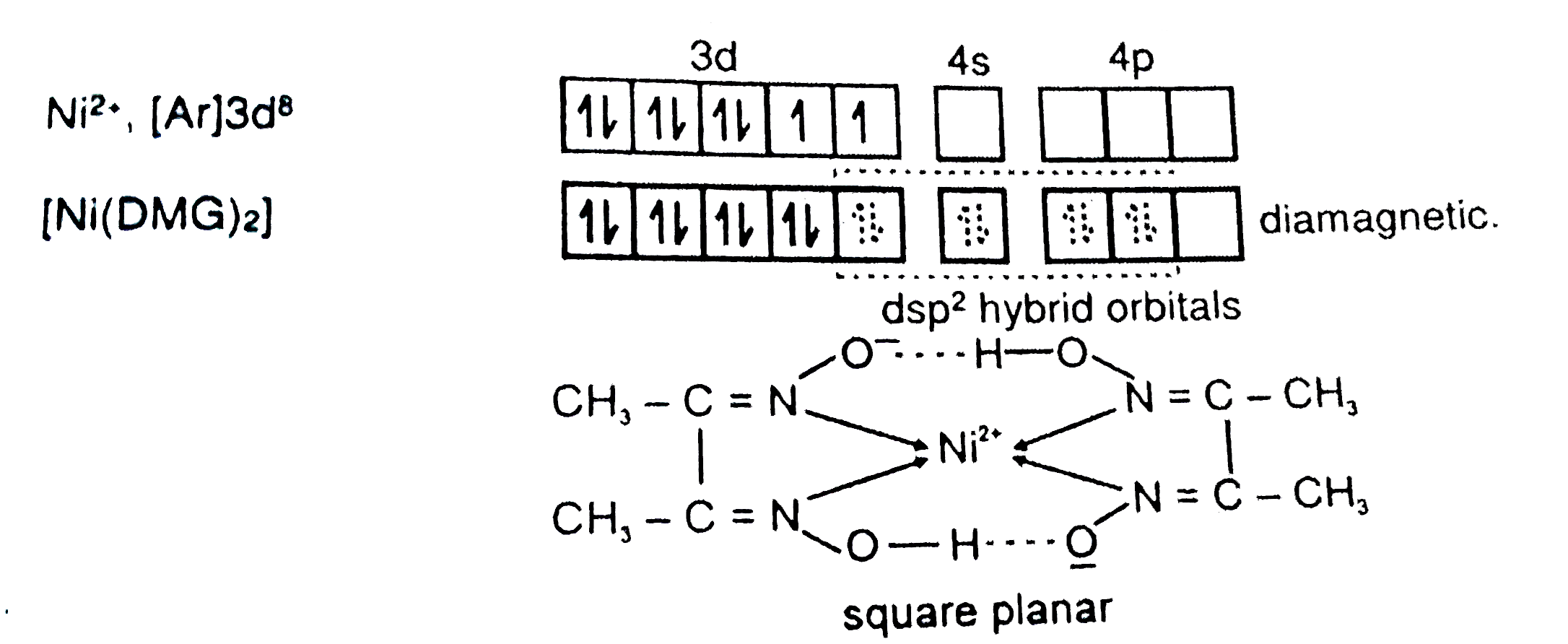
27. DMG +NiCl2+NH4OH makes Complex a+ NH4Cl+H2O. What is complex a and find the hybridisation magnetic character and Oxidation state of Nickel in complex a ?

E735: Complex Ions and Precipitates – Nickel(II) compounds | Lecture Demonstration Manual General Chemistry | University of Colorado Boulder
29. NiCl2 + NH4OH + dimethylglyoxime ——> A ( complex ) Incorrect statement for complex A is /are 1 Coordination number of metal ion is 4 2 Two five membered and two
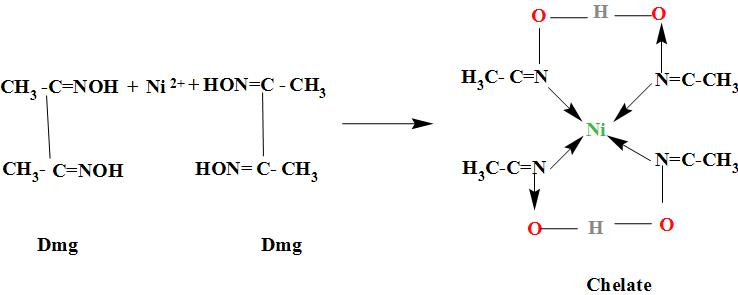
When dimethylglyoxime solution is added to an aqueous solution of nickel (II) chloride followed by ammonium hydroxide, then which of the following statements are incorrect?This question has multiple correct questions(a) No precipitate

NiCl_2 in the presence of dimethyl glycoxime (DMG) gives a complex which precipitates in the presence of NH_4OH, giving a bright red color.Draw its structure and show H bonding.






/fusion-NH4OH-generation-system-process-flow-diagram.png?width=710&name=fusion-NH4OH-generation-system-process-flow-diagram.png)